 Sleuthing: Using Blood Values to determine the Cause of Acidosis or Alkalosis
Sleuthing: Using Blood Values to determine the Cause of Acidosis or Alkalosis
- Note the pH. This tells you whether the person is in acidosis (pH < 7.35) or alkalosis (pH > 7.45); but it does not tell you the cause.
- Next, check the PCO2 to see if this is the cause of the acid-base imbalance. Because the respiratory system is a fast-acting system, an excessively high or low PCO2 may indicate either that the condition is respiratory system—caused or that the respiratory system is compensating. For example, if the pH indicates acidosis and:
- The PCO2 is over 45 mm Hg, the respiratory system is the cause of the problem and the condition is a respiratory acidosis.
- The PCO2 is below normal limits (below 35 mmHg), the respiratory system is not the cause but is compensating.
- The PCO2 is within normal limits; the condition is neither caused nor compensated by the respiratory system.
- Check the bicarbonate level. If step 2 proves that the respiratory system is not responsible for the imbalance, then the condition is metabolic and should be reflected in increased or decreased bicarbonate levels. Metabolic acidosis is indicated by HCO3– values below 22 mEq/L, and metabolic alkalosis by values over 26 mEq/L. Notice that whereas PCO2 vary inversely with blood pH (PCO2 rises as blood pH falls), HCO3– levels vary directly with blood pH (increased HCO3– results in increased pH). Beyond this bare-bones approach there is something else to consider when you are assessing acid-base problems. If an imbalance is fully compensated, the pH may be normal even when the pH is normal, carefully scrutinize the PCO2 or HCO3– values for clues to what imbalance may be occurring.
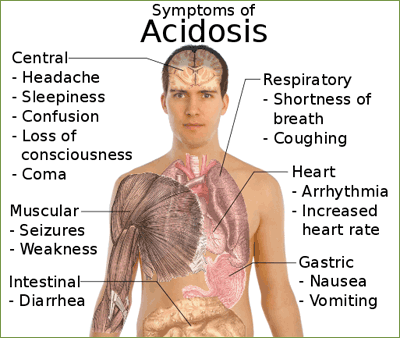
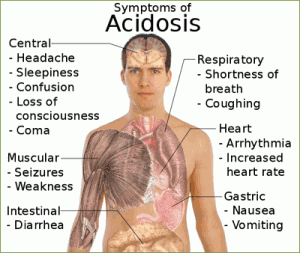
Causes and Consequences of Acid-Base imbalances
Metabolic acidosis:
- Uncompensated (uncorrected) HCO3– < 22 mEq/L; pH < 7.4
- Severe diarrhea: Bicarbonate-rich intestinal (and pancreatic) secretions rushed through digestive tract before their solutes can be reabsorbed; bicarbonate ions are replaced by renal mechanisms that generate new bicarbonate ions.
- Renal disease: failure of the kidneys to rid body of acids formed by normal metabolic processes.
- Untreated diabetes mellitus: lack of insulin or inability of tissue cells to respond to insulin, resulting in inability to use glucose; fats are used as primary energy fuel, and ketoacidosis occurs.
- Starvation: Lack of dietary nutrients for cellular fuels, body proteins and fat reserves are used for energy—both yield acidic metabolites as they are broken down for energy.
- High ECF potassium concentrations: Potassium ions compete with H+ for secretion in renal tubules; when ECF levels of K+ are high, H+ secretion is inhibited.
Metabolic alkalosis:
- Uncompensated (HCO3– >26 mEq/L; pH > 7.4)
- Vomiting or gastric suctioning: loss of stomach HCl requires that H+ be withdrawn from blood to replace stomach acids; thus H+ decreases and HCO3– proportionally.
- Selected diuretics: cause K+ depletion and H2O loss. Low K+ directly stimulates the tubule cells to secrete H+. Reduced blood volume elicits the renin-angiotensin mechanism, which stimulates Na+ reabsorption and H+ secretion.
- Ingestion of excessive sodium bicarbonate (antacid): bicarbonate moves easily into ECF, where it enhances natural alkaline reserve.
- Constipation: prolonged retention of feces, resulting in increased amounts of HCO3– being reabsorbed.
- Excessive aldosterone: (adrenal tumors) promotes excessive reabsorption of Na+, which pulls increased amount of H+ into urine. Hypovolemia promotes the same relative effect because aldosterone secretion is increased to enhance Na+ (and H2O) reabsorption.
Respiratory acidosis:
- Uncompensated (PCO2 >45 mm Hg; pH <7.4)
- Impaired gas exchange or lung ventilation (chronic bronchitis, cystic fibrosis, emphysema): Increased airway resistance and decreased expiratory air flow, leading to retention of carbon dioxide.
- Rapid, shallow breathing: Tidal volume markedly reduced.
- Narcotic or barbiturate overdose or injury to the brain stem: depression of respiratory centers, resulting in hypoventilation and respiratory arrest.
Respiratory alkalosis:
- Uncompensated (PCO2 < 35 mm Hg; pH > 7.4)
- Direct cause is always hyperventilation: hyperventilation is pain/anxiety, asthma, pneumonia, and at high altitude represents effort to raise PO2 at the expense of excessive carbon dioxide excretion.
- Brain injury or tumor: abnormality of respiratory controls.